Detecting multi-antigen serological responses with EYRAbeads
Published: December 12, 2025
Updated: December 16, 2025
3 minute read
Authored by: Tyler Sandberg
Understanding how the immune system responds to vaccination often requires looking beyond a single antigen-specific antibody. Many pathogens and vaccines trigger complex, multi-antigen antibody responses that shape long-term protection. Traditional ELISAs only allow measurement of a single antigen at a time, forcing researchers to prioritize which part of the response they want to track.
EYRAbeads change that.
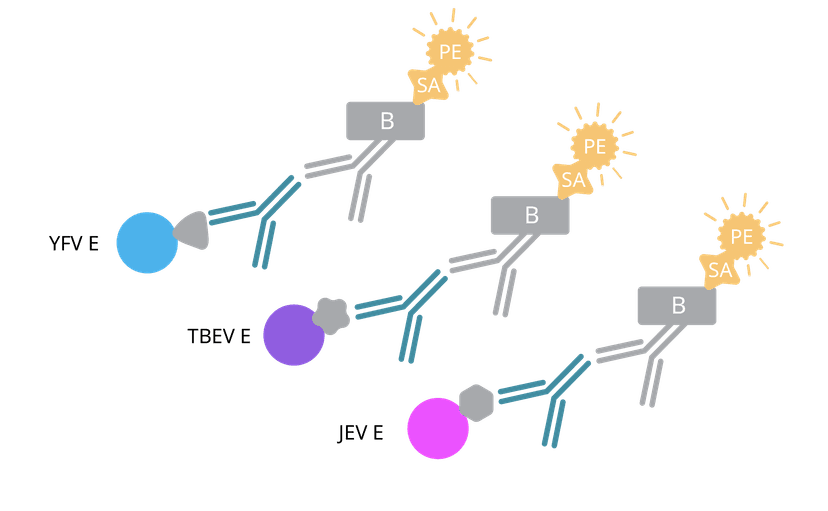
These dye-stabilized carboxylated magnetic beads can be coupled with virtually any antigen, enabling the simultaneous measurement of multiple antibody specificities from the same serum sample. In this proof-of-concept project, we set out to show how EYRAbeads can reliably detect antibodies against three different flaviviruses within a single assay:
- Yellow fever virus (YFV)
- Tick-borne encephalitis virus (TBEV)
- Japanese encephalitis virus (JEV)
These viruses correspond to the vaccines administered in a clinical trial conducted by one of our collaborators at the Karolinska Institutet. By multiplexing with EYRAbeads, we were able to use far less serum than three separate ELISAs would require while still capturing the same immunological insights.
Building the custom multiplex serology assay
Each viral Envelope (E) protein was conjugated to a unique EYRAbead region (three in total). To determine the optimal antigen density, several coupling concentrations were tested on 4 million EYRAbeads per antigen. This quick titration step can typically be completed in a single day and ensures that each bead population is properly saturated with its antigen at an ideal density that gives a strong signal with minimal background noise. Before using real vaccinee samples, we confirmed the ideal antigen-EYRAbead concentration using a pan-specific IgG in the EYRAplex assay.
Once optimal conjugations were determined, the E-protein-coupled EYRAbeads were tested with human serum collected before and after vaccination. The workflow followed the familiar EYRAplex protocol:
- 2,000 beads per antigen per well
- Multiple serum dilutions
- Detection using anti-human IgG (MT78/145, biotin) and streptavidin-PE
Because each antigen is coupled to its own bead region, all three antibody responses could be measured simultaneously and detected using PE fluorescence on Mabtech EYRA. Creating the custom assay was as simple as selecting the appropriate Bead IDs in the Opal software and clicking, "Read".
Our results
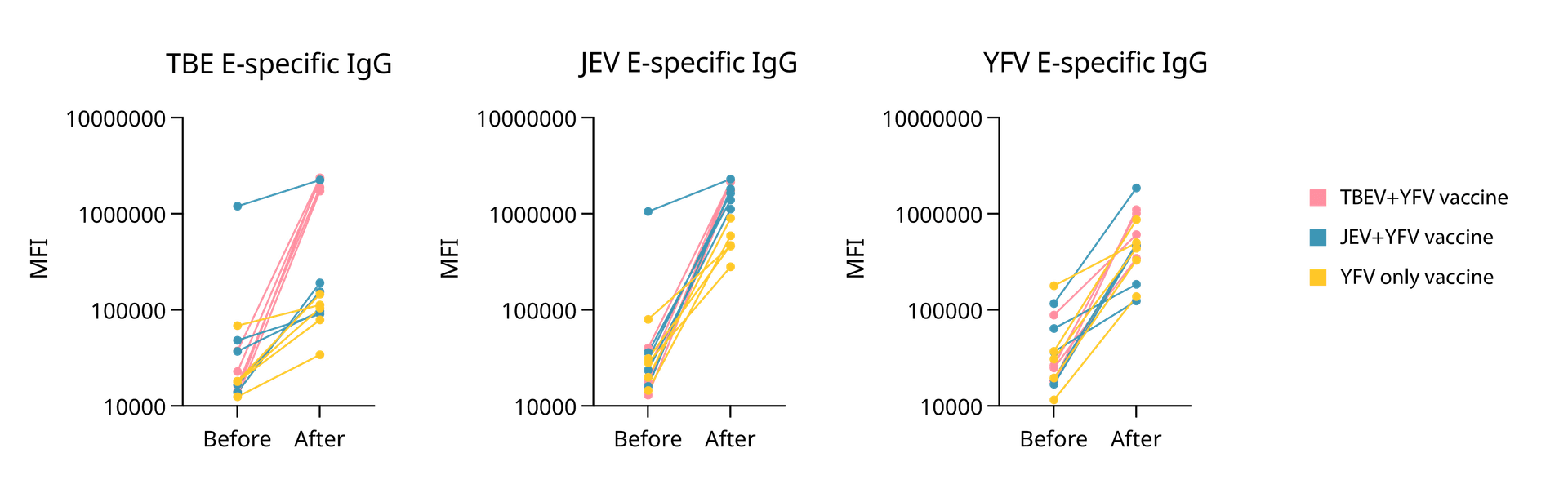
We clearly detected flavivirus-specific IgG responses in post-vaccination samples, confirming that EYRAbeads are a helpful tool for antigen-specific serology. Interestingly, some cross-reactive binding was observed between viruses, suggesting that conserved regions within flavivirus Envelope proteins may drive shared IgG responses. This is exactly the type of biological nuance multiplexing can help reveal.
Median fluorescent intensities (MFI) of serum IgG binding to Envelope proteins from TBEV, JEV, and YFV measured using antigen-coupled EYRAbeads before and after vaccination. Each line represents an individual donor, with paired measurements connecting pre-vaccination and post-vaccination samples. Donors are grouped by vaccination regimen: TBEV+YFV (pink), JEV+YFV (blue), and YFV-only (yellow).
Since EYRAbeads can be conjugated with nearly any protein or peptide, researchers could explore this further by:
- Coupling more granular Envelope epitopes to separate Bead IDs
- Assessing cross-reactivity at a higher-resolution
- Expanding to IgM, IgA, or IgG1-4 detection to uncover class- or subclass-specific patterns
- Profiling broader immune responses across multiple pathogens or vaccine candidates
The sky is truly the limit. EYRAbeads give researchers the flexibility to build exactly the serology assay they need from exploratory experiments to large-scale vaccine clinical trials and more.