Immunoassays for immunotherapy research
Published: February 23, 2024
Updated: June 11, 2025
10 minute read
Authored by: Tyler Sandberg
ELISpot and FluoroSpot are perfect potency assays to use during the development of immunotherapies as well as cell and gene therapies, offering critical insights into CAR-T cell functionality, TIL activity, cancer vaccine efficacy, and gene therapy using AAV or other viral vectors. With the FDA's recommendations on using cytokine secretion assays, ensure your therapy's path to success is clearly mapped with ELISpot and FluoroSpot.
New cell and gene therapies (CGT), advanced therapy medicinal products (ATMP), and immunotherapies are being developed faster than ever, treating a wide range of diseases, from genetic disorders to cancers, by modifying a patient’s own cells or even introducing new genes to restore normal function. The FDA’s guidelines for CGT and ATMP evaluation stress the importance of comprehensive immune analysis, highlighting cytokine secretion assays as a necessary tool for assessing the efficacy and safety of these therapies. While flow cytometry offers valuable insights into cell populations and their functions, it may not fully capture the nuanced cytokine profiles elicited by CGT and ATMP. Cytokine secretion assays, including ELISpot and FluoroSpot, can fill this gap and provide a detailed map of specific cytokine secretion responses at the cellular level. Keep reading to learn how ELISpot and FluoroSpot can provide valuable insights into CAR-T cell therapies, TILs, viral vector-based gene therapies, and cancer vaccines.
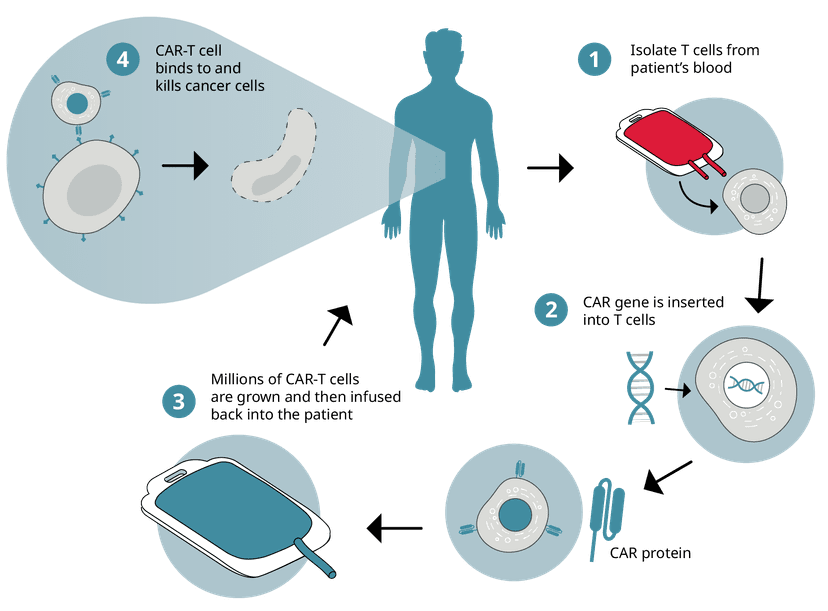
CAR-T cell therapy
Collected immune cells undergo genetic engineering in the lab introducing the chimeric antigen receptor (CAR) gene to give them the ability to recognize cancer cells and enhance their capacity to kill them. These new CAR-T cells are then expanded in culture before being infused back into the patient.
CAR-T cell therapies harness the power of a patient's immune system to fight cancer. By reprogramming a patient’s T cells to target specific cancer antigens, this personalized approach has led to six different FDA-approved therapies against blood cancers like leukemia and lymphoma, offering new strategies for treating previously intractable cancers. In line with FDA considerations, cytokine release assays are essential in the development of CAR-T cell products, ensuring safety and efficacy in these innovative treatments. This therapy not only signifies a major leap in our fight against cancer but also demonstrates the promise of precision medicine to provide patient-specific, more effective, and less invasive cancer therapies.
So how is CAR-T cell functionality tested during development? Both in vitro and in vivo assessments are important to ensure efficacy against cancer cells. In vitro potency assays like cytotoxicity or “killing assays”, chromium release assays, and flow cytometry-based methods can test if CAR-T cells induce target cell killing. Ideally, you want to test if CAR-T cell activation occurs in the presence of target cancer cells through degranulation and cytokine release. This is where ELISpot and FluoroSpot can be key assays due to their extreme sensitivity in detecting IFN-γ, granzyme B, perforin, IL-2, or TNF-α-secreting cells. We even developed a FluoroSpot kit just for this purpose: FluoroSpot Path: Human immunotherapy potency (3‑color).
In vivo, CAR-T cells' therapeutic impact is assessed not only through tumor size reduction, survival rates, and phenotypical analyses using flow cytometry but also with re-challenge responses utilizing ELISpot and FluoroSpot. CAR-T cells are isolated from animal models or patients and re-challenged with target cancer cells to assess degranulation and cytokine release as before. Together, these techniques provide a comprehensive picture of CAR-T cell functionality guiding refinements of the therapy for clinical success.
CAR-T cell functionality can be assessed with FluoroSpot by directly challenging the cells with cancer cells expressing the tumor-associated antigen (TAA) or with the TAA itself. Functioning cells will secrete the analytes of interest (IFN- γ, granzyme B, perforin, IL-2, or TNF-α) that can be detected with FluoroSpot.
Once the CAR-T cells are deemed functional, it's time to assess their efficacy in a clinical trial setting. Following infusion, CAR-T cells can be collected with PBMC samples and their functionality can again be assessed by coculturing with target cells in ELISpot or FluoroSpot. This will inform if the infusion of CAR-T cells into patients or animal models affects their functionality. Additionally, adverse effect monitoring is critical for assessing the safety and tolerability of the therapy. Here, cytokine storms can be an unfortunate adverse event and can easily be assessed by testing patient or animal serum levels for IL-6 using standardized ELISA kits. Additionally, the therapy should be free of pyrogens and can be evaluated using several MAT assays (IL-6 ELISA, IL-1b ELISA, and IL-6/IL-1b FluoroSpot).
Click the following link to read a how one research group found the optimal FluoroSpot analyte combination for assessing CD19 targeted CAR-T cells.
Alongside CAR-T cell therapies, TCR (T-cell receptor) therapies are emerging as a powerful method for targeting a wider array of cancer antigens with enhanced specificity. These therapies modify a patient's T cells to express a specific TCR against cancer antigens, broadening their ability to fight the cancer. Additionally, engineered NK cells, known as CAR-NK therapies, are gaining traction as they can target tumors without the need for prior sensitization, potentially reducing the risk of cytokine release syndrome. Both TCR and CAR-NK therapies can be assessed using similar in vitro and in vivo methods as CAR-T cells, ensuring their efficacy and safety profiles are thoroughly evaluated.
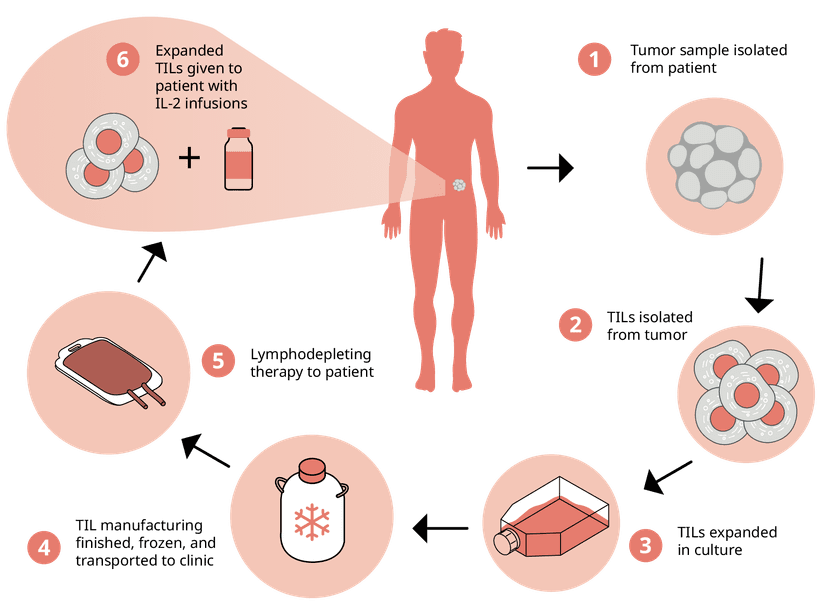
Tumor-infiltrating lymphocytes (TILs)
Building on the personalized nature of cell-based immunotherapies, Tumor Infiltrating Lymphocytes (TILs) offer another frontier in cancer treatment. These cells, which are isolated directly from a patient's tumor, are naturally proficient at recognizing cancer cells meaning they don’t need genetic engineering like with CAR-T cells. Like with CAR-T cells though, they are expanded into the millions and reinfused to boost the immune response against tumors. Recently, the FDA approved the first TIL therapy, Lifileucel, against the solid tumor of skin cancer melanoma. And with the FDA’s emphasis on thorough immune response assessments, cytokine secretion assays like ELISpot and FluoroSpot are perfect for measuring the functionality, activity, and potency of the isolated TILs.
Tumor-infiltrating lymphocytes (TILs) are harvested directly from a patient's tumor tissue. After being isolated from the extracted tumor, TILs are cultivated to increase their numbers significantly and then reintroduced into the patient's body.
So how can ELISpot or FluoroSpot be used to monitor TIL potency during manufacturing? There are three main questions that TIL researchers would like to assess when evaluating TIL efficacy. The first is if the isolated TILs can be activated against autologous tumors. Here, cultured TILs are exposed to the cancer cells and their responses are measured by capturing relevant cytokines and analytes that can kill the target cells using ELISpot or multiplexed with FluoroSpot (IFN-γ, granzyme B, perforin, IL-2, or TNF-α). We developed a FluoroSpot kit that's perfectly suited to evaluate your TIL product: FluoroSpot Path: Human immunotherapy potency (3‑color). Secondly, changes in TIL composition need to be assessed following either rapid expansion (REP) or bulk culturing processes. Cells from both manufacturing strategies are stimulated with tumor-associated peptides, and their responding secretory profiles are evaluated with ELISpot or FluoroSpot. And finally, as with CAR-T cells, pyrogen testing is essential using MAT assay strategies (IL-6 ELISA, IL-1b ELISA, IL-6/IL-1b FluoroSpot).
TIL functionality can be assessed with FluoroSpot by directly challenging the cells with autologous cancer cells expressing the tumor-specific antigen (TSA) or with TSA peptide pools. Functioning cells will secrete the analytes of interest (IFN- γ, granzyme B, perforin, IL-2, or TNF-α) that can be detected with FluoroSpot.
An excellent paper from 2018 showcased ELISpot as a key screening tool in their assessment of TILs used in breast cancer therapy. Read about it here!
Cancer vaccines
Cancer vaccines are designed to elicit a robust immune response against tumors and are paving the way for novel cancer treatments. These vaccines come in various forms:
- Nucleic acid-based vaccines, utilizing DNA or RNA, instruct the patient's cells to produce tumor antigens triggering a targeted immune reaction
- Cell-based vaccines direct the immune response against cancer cell antigens
- Viral-based vaccines use modified viruses to deliver cancer antigens
- Peptide-based vaccines leverage short sequences of proteins from cancer cells to stimulate an immune response
We've mentioned both TAAs and TSAs as potential targets in previous therapies, and it's no different with cancer vaccines. However, a shift towards TSAs has emerged due to their higher immunogenicity and since these are not present in healthy tissue avoiding undesired tissue damage. These cancer vaccines signify a multidimensional approach to stimulate the body's defenses, marking a new era in the fight against cancer. With each vaccine type comes unique challenges in development and assessment, where sensitive tools like ELISpot and FluoroSpot assays are invaluable in evaluating their efficacy and shaping their therapeutic potential.
Researchers gather sequencing data to identify potential TSAs that are then used in viral vector-, peptide-, or DNA/RNA-based vaccines which are manufactured and administered to patients to elicit cancer-specific immune responses.
Assessing robust, cancer-specific immune responses in patients is streamlined with ELISpot and FluoroSpot assays. Samples of PBMCs collected before and after vaccination, including booster doses, can be analyzed directly or after sorting into CD8 or CD4 T cell subsets. These cells, when stimulated with TSA-derived peptide pools, reveal key immune responses through the secretion of IFN-γ, TNF-α, IL-2, granzyme B, and perforin, easily measured by these assays. Why not try our FluoroSpot Path: Human immune monitoring (3‑color) kit specifically designed to evaluate important analyte secretion associated with vaccine-induced responses?
Isolated PBMCs can be stimulated with TSA-derived peptide pools to measure cancer-specific T cell responses induced by the cancer vaccine.
Last year, researchers published their findings on a personalized neoantigen pancreatic cancer vaccine where they used ELISpot as a primary readout in assessment of neoantigen-specific T cells.
Gene therapy using viral vectors
Viral vector-based gene therapies start with the design of a plasmid containing the transgene for the missing or defective gene. The viral vector is manufactured containing the transgene and given to the patient to restore the production of the specific protein and normal function.
Gene therapies are innovative strategies used to counteract inherited or complex diseases. The goal is to replace the mutant or missing gene through gene correction, gene replacement or augmentation, or gene silencing. We’ll focus here on in vivo therapies where the gene of interest is delivered to patients via a viral delivery vector. Adeno-associated virus (AAV) gene therapy represents the most common viral vector gene therapy. These therapies utilize AAV vectors that are engineered to be non-pathogenic and favored for their targeting precision and stable gene delivery. AAV gene therapies introduce functional genes called transgenes to compensate for defective or missing ones, offering the potential to correct disorders at their genetic roots. With several FDA-approved AAV therapies already available, they’ve set a precedent in the field of genetic medicine, opening doors to curing hereditary conditions that were once considered life-long challenges. The role of immune response assessment becomes increasingly important, with ELISpot and FluoroSpot assays providing key insights into the immune system's interaction with these novel therapeutics.
Traditionally, immune response measurement to AAV gene therapies has focused on detecting AAV- or transgene protein-specific antibodies through IgG ELISA kits or neutralization assays. However, the evaluation of T cell-mediated immunity is equally important and provides additional important immune insights. By analyzing PBMC samples collected pre- and post-treatment using peptide pools derived from both the AAV vector and the transgene, ELISpot and FluoroSpot assays allow for a sensitive and robust assessment of cellular immune responses. Ideally, for a successful therapy, one would expect minimal to no spot formation in assays targeting IFN-γ, TNF-α, IL-2, and granzyme B. Additionally, innate immune responses, such as type I interferon secretion (IFN-α or IFN-β), can be quantified, providing a comprehensive profile of the immune system's engagement with the therapy.
Immune cells can be isolated before and after AAV gene therapy to assess their immunogenicity using AAV or transgene-derive peptide pools. Additionally, innate responses to the AAV and transgene themselves are tested, specifically plasmacytoid dendritic cells (pDCs) and other innate drivers of IFN type I responses.
We wrote a highlighted research post about a recent paper comparing ELISpot and FluoroSpot with flow cytometry methods when assessing AAV gene therapies. The post focuses on the methodologies themselves, but the associated paper is an excellent source for inspiring how you can set up your AAV gene therapy assessment with ELISpot of FluoroSpot.
Checkpoint blockade inhibitors
Checkpoint inhibitors are a type of immunotherapy that works by blocking proteins that inhibit the immune system from effectively attacking cancer cells. These therapies are often categorized as both monoclonal antibodies and targeted treatments, as they can directly target specific immune checkpoints like PD-1 or CTLA-4, which are responsible for dampening immune responses.
ELISpot and FluoroSpot assays can be key tools in evaluating the efficacy of checkpoint inhibitors. By measuring cytokine secretion, such as IFN-γ or TNF-α, these assays can provide direct evidence of enhanced T cell activity in response to a checkpoint blockade therapy. FluoroSpot, with its ability to detect multiple cytokines simultaneously, can further characterize the polyfunctional nature of T cells activated by checkpoint inhibitors, providing a more comprehensive understanding of the immune response.
This kind of immune monitoring is critical for assessing the potency and mechanism of action of checkpoint inhibitors in both pre-clinical and clinical settings. For more on this, you can explore how Nobel Laureates James P. Allison and Prof. Tasuku Honjo used Mabtech’s ELISpot technology.
We hope this overview has demonstrated the indispensable role of ELISpot and FluoroSpot assays in the development and evaluation of different CGTs. These assays can provide in-depth immune response data, essential for advancing your clinical trials and contributing to the success of emerging therapies. Interested in learning more about how ELISpot or FluoroSpot could enhance your CGT evaluation efforts? Please contact us! We'd love to hear from you.
Want to learn more? Watch our recent webinar on using ELISpot and FluoroSpot in cell and gene therapy!