7 common ELISpot errors and how to avoid them
Published: November 7, 2022
Updated: September 29, 2025
5 minute read
Authored by: Jens Gertow
We’ve been doing ELISpot since the 80’s and during all those years, we’ve seen ELISpot work great and, unfortunately, sometimes not so great. Luckily for you, we’ve already come across all the mistakes and have learned from them to make the best kits available. Here we’ll discuss 7 of the most common mistakes we’ve encountered in ELISpot and how to avoid them.
1. No spots at all
It’s not always easy to figure out why there aren’t any spots in an ELISpot plate, but below are the most common reasons:
| Mistake | Solution |
|---|---|
| ELISA substrate was used | Precipitating ELISpot substrate should be used |
| Low viability in sample | Cells should have at least 89% viability for measuring reliable Ag-specific responses |
| Suboptimal incubation time | Different analytes require different incubation times. Check the data sheet for recommended incubation times! |
| Too many cells – creates confluent coating of the well | We typically recommend 250,000 cells for detecting Ag-specific T cell responses. Be sure to check the data sheet for recommendations. |
2. Blank wells? How’d that happen?
In our experience, blank wells occur most often when some reagent wasn’t added. We’re all human. It happens to the best of us!
Another common mistake deals with pipetting errors. Bubble formation in the wells can block reagents from reaching the bottom. The solution is to inspect the wells after addition of each reagent removing bubbles and looking for wells that don’t look like the others. Be sure not to touch the inner wall of the wells with the pipette tip.
Luckily, blank wells may be able to be “rescued” with a recovery protocol below:
- Wash the blank wells 5x with 200ul PBS.
- Add detection antibody at 1ug/ml in PBS containing 0.5% FCS and incubate for 2h.
- Wash the blank wells 5x with 200ul PBS.
- Add SA-ALP in PBS containing 0.5% FCS and incubate for 1h.
- Wash the blank wells 5x with 200ul PBS.
- Add substrate and incubate for 10min.
3. Patchy background
Sometimes, a patchy background shows up in ELISpot wells and this can happen for a couple of reasons.
- Improper ethanol treatment and washing steps are common culprits. Consult the datasheet and protocol for proper ethanol concentration and washing instructions.
- Cell debris is another common cause of patchy backgrounds. Be sure to use cells with high viability and handle them properly. You can learn how we attain >95% cell viability every time with our optimizing cell handling protocols here.
- Precipitates can form after prolonged substrate storage, leading to patchy backdrounds. The solution here is easy though. Filter the substrate! Prior to adding the substrate to the wells, filter it through a 0.45 µm (syringe) filter to minimize patchy precipitation.
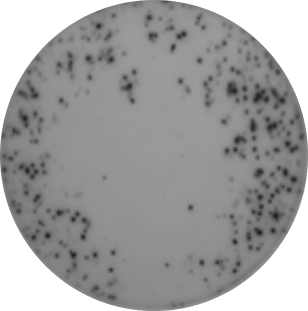
4. Ring of spots – only on the edges
It’s not uncommon to see ELISpot wells with uneven distribution of cells. To prevent this, we suggest adding reagents and cells in the following order:
First add 50 µl of stimuli or medium control and then add 50 µl of your cell suspension. This prevents the cells from being “pushed” around like it would if stimulant or media is added after the cells.
Stimuli first, then cells
5. I saw spots, now they're gone
In rare cases, when using tap water for the final plate washing, the normally distinct, blue TMB spots may change color, first to green, then to yellow, and finally disappear. This is caused by a high content of ionic compounds in the water. If unsure about the water quality, we recommend plates to be rinsed with deionized water to stop the substrate reaction.
Before storage
After storage
We recommend the following:
- Make sure all the PBS from the last washing step is gone before adding TMB.
- Stop the enzymatic reaction with deionized water.
- Easiest of all: switch to ALP detection instead of HRP. This yellow phenomenon only occurs in HRP ELISpot.
6. White spots on the membrane
Ever find some white spots on the ELISpot membrane? We’ve been there. These spots are often caused by solvents, like Tween in a washing buffer or DMSO in the antigen stimulation suspension. Solvents like these can damage the PVDF membrane as well as solubilize cell membranes leaving cell debris on the membrane that can result in strange membrane appearances.
It’s quite simple to avoid these white spots though. Never use Tween in your ELISpot assays and keep DMSO at a concentration less than 0.5% in the ELISpot wells.
7. Dark ELISpot membrane
Sometimes the ELISpot membrane can appear dark. There are a couple reasons for this:
- Human serum in cell culture medium is a common culprit to a dark well. Human serum can contain cytokines that bind to the capture antibodies and even heterophilic antibodies. Switching to FBS could be just the solution you need!
- Tween is another common reason for dark backgrounds. Tween can damage the membrane which is why we never recommend using it in your ELISpot assay.
- Previously activated cells can already be expressing cytokines of interest in the supernatant prior to adding them to the ELISpot plate. This can lead to general darkening of the membrane. It’s always a good idea to wash the cells and resuspend in fresh medium and move them directly to the ELISpot plate.
- High DMSO concentrations can also lead to dark backgrounds in the wells. DMSO in high concentrations can cause the ELISpot membrane to leak, drawing in the detection antibodies deep into the membrane. It can be difficult to wash them away leading to a darker background upon developing the plate. Prevent this by keeping DMSO concentrations under 0.5%.
Beyond the 7 above, other issues can often be traced back to cell quality. For optimal cell handling, be sure to read our tutorials!
Do you other issues? Check out our Community forum!