Isolation, freezing, and thawing of PBMCs
Published: September 2, 2022
Updated: November 27, 2025
8 minute read
Authored by: Jens Gertow
Peripheral blood mononuclear cells (PBMCs) are, at least for human samples, the most commonly used cells in ELISpot and FluoroSpot assays.
PBMCs can easily be isolated from blood by density centrifugation using e.g., Ficoll-Paque. The cells can be used directly after isolation, or more conveniently, cryopreserved and used at a later timepoint.
This is a detailed guide on how to best isolate, freeze, and thaw PBMCs.
Isolating PMBCs
For collection of whole blood, a variety of blood collection tubes with different anticoagulants are available on the market. In our experience, citrate and heparin tubes are best suited for ELISpot.
If a large number of cells is required, PBMCs can also be prepared from a buffy coat, which is a concentrate of leukocytes obtained after removal of plasma and most of the red blood cells.
Regardless of anti-coagulant or isolation method, ensure that PBMCs are isolated within 8 hours of blood draw to reduce the risk of granulocyte contamination that can negatively impact your ELISpot or FluoroSpot results.
Materials
- Blood sample (whole blood or buffy coat)
- Ficoll-Paque, sterile
- PBS, sterile or medium without serum (e.g., RPMI 1640)
- Polypropylene centrifuge tubes: 50 ml and/or 15 ml, sterile
- Pipettes and pipette boy
- Pasteur pipettes (sterile; plastic or glass)
- Automatic pipettes and sterile tips
- Centrifuge at room temperature
Before you begin: Ensure that all reagents are adjusted to room temperature. Work under aseptic conditions and according to local regulations for biological hazards
Steps
1. Whole blood: Dilute the blood 2x with PBS (e.g., 10 ml blood + 10 ml PBS). Buffy coat: Cells are denser in a buffy coat compared to whole blood and should therefore be diluted more (around 3x).
2. Prepare centrifuge tubes with Ficoll-Paque. Depending on the blood volume, use either 15 ml tubes or 50 ml tubes. If using 15 ml tubes, add 5 ml of Ficoll-Paque. If using 50 ml tubes, add 15 ml of Ficoll-Paque.
3. Gently add the diluted blood on top of the Ficoll-Paque layer without disturbing the interface. For 15 ml tubes, add 8 ml of diluted blood and for 50 ml tubes, add 25 ml of diluted blood.
Tip: Hold the pipette against the tube wall while tilting the tube.
4. Centrifuge at 400 x g, without brake, for 30 minutes at room temperature.
5. After centrifugation, a white-grayish layer consisting of mononuclear cells will be found on top of the Ficoll-Paque. Carefully collect the mononuclear cells using a Pasteur pipette and transfer to a new centrifuge tube. Alternatively, first remove some of the plasma layer before collecting the mononuclear cells. Do not pool cells from more than two Ficoll tubes and keep the volume (below half the tube volume). Fill up this new tube with either PBS or medium without serum.
6. Wash the cells by centrifuging the new tube for 10 minutes at 400 x g.
7. Discard the supernatant, resuspend the pellet, and add PBS or medium without serum.
Tip: When resuspending cells, first tap the tube to make the cell pellet less compact. Then add 1-2 ml buffer/medium and slowly pipette the solution up and down before filling the tube.
8. Wash the cells by centrifuging the new tube for 10 minutes at 400 x g.
9. Discard the supernatant, resuspend the pellet, and add medium without serum.
10. Note the volume and take a small aliquot to count the cells.
11. Wash the cells by centrifuging for 10 minutes at 400 x g.
Tip: Count the cells during this step.
Contaminating red blood cells (RBC) and granulocytes can be present after the Ficoll separation. If desired, depletion of these can be done with commercially available reagents. (Google for example "RBC lysis buffer" and "granulocyte depletion".)
12. If you want to use the cells right away
Discard the supernatant and resuspend the cell pellet in appropriate cell culture medium by gently pipetting up and down. The PBMCs are now ready to be used for cell-based immunoassays such as ELISpot, FluoroSpot, and flow cytometry.
If you want to cryopreserve the cells
Continue to section “Freezing PBMCs”
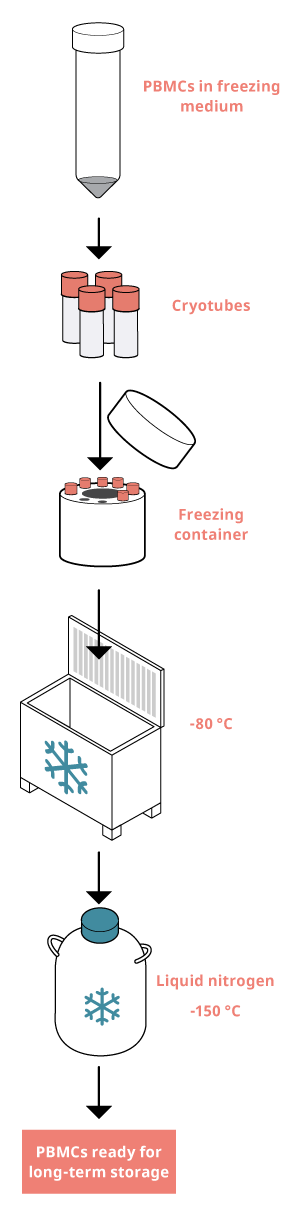
Freezing PBMCs
Materials
- Freezing medium: RPMI 1640 (with 2mM L-glutamine) + 20% FCS and 10% DMSO. If your cells are adapted to serum-free medium, use 7% DMSO
- Cryotubes, e.g., 1.8 ml
- Polypropylene centrifuge tubes: 50 ml and/or 15 ml, sterile
- Freezing container e.g., “CoolCell” or “Mr. Frosty
- Automatic pipettes and sterile tips
- -80 °C freezer
- -150 °C freezer or liquid nitrogen tank
Before you begin: Label cryotubes and make sure to have the freezing container(s) ready. Leaving cells for too long in the freezing medium at room temperature can negatively affect cell viability.
Steps
1. Discard the supernatant (from step 11 in the previous section) and dilute the cells in freezing medium to a concentration of 5 to 25 million cells/ml. If you are using serum-free freezing medium, aim instead for a cell concentration of 1 million cells/ml.
2. Aliquot e.g., 1 ml in each cryotube and transfer the cryotubes to the freezing container. Make sure to keep your cells in suspension by continuous resuspension.
Tip: For 1.8 ml cryotubes, aliquot 1 ml cell suspension per tube. For other sizes of cryotubes, make sure to aliquot less than the maximum volume, as the liquid expands during freezing.
3. Quickly place the freezing container in a -80 °C freezer, and store for a minimum of 4 hours, and a maximum of 1 week.
4. Transfer the cryotubes from the freezing container to a -150 °C freezer or liquid nitrogen tank for long-term storage.
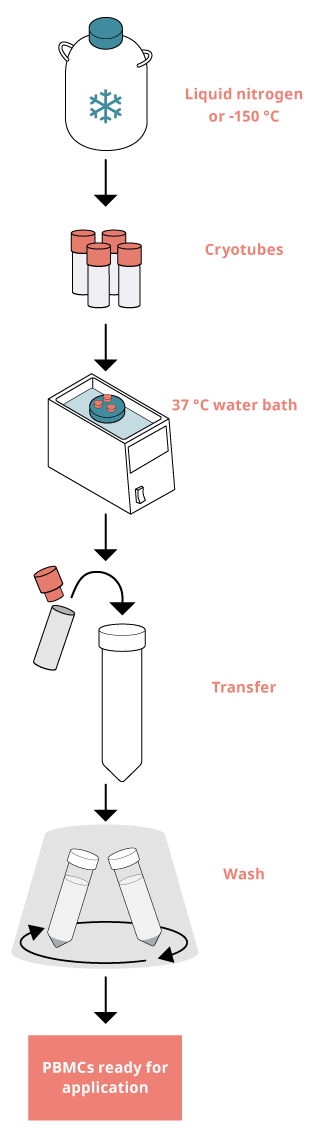
Thawing PBMCs
Materials
- Cell culture medium: RPMI 1640 (with 2mM L-glutamine) + 10% FCS, 10 mM HEPES and 100 µg/ml penicillin + 100 µg/ml streptomycin
- Polypropylene centrifuge tubes: 50 ml and/or 15 ml, sterile
- Pipettes and pipette boy
- Automatic pipettes and sterile tips
- Centrifuge at room temperature
- Water bath at 37 °C
- CO2 incubator at 37 °C
Before you begin: Heat a water bath to 37 °C. Pre-heat the cell culture medium to be used for step 2. It's fine if the medium used in later washing steps is of room temperature.
Steps
1. Transfer the cryotube rapidly from the freeze storage to a 37 °C water bath and thaw the cells until only a small ice crystal remains.
Tip: If you intend to thaw multiple cryotubes, only thaw a few at a time. The freezing medium contains DMSO which is toxic to cells so proceed to step 2 as soon as possible.
2. Slowly add 0.5-1 ml of the pre-heated cell culture medium to the cryotube, resuspend and transfer the cells from the cryotube to a 15 ml tube. Rinse the cryotube with 1 ml of cell culture medium and transfer it to the 15 ml tube. Fill the tube with cell culture medium.
3. Wash by centrifugation for 10 minutes at 300 x g.
4. Discard the supernatant and tap the tube to make the pellet less compact. Resuspend the cells in 1 ml of cell culture medium by slowly pipetting up and down. Add cell culture medium to a total volume of 15 ml.
5. Centrifuge for 10 minutes at 300 x g.
6. Discard the supernatant and resuspend the pellet in 1 ml of cell culture medium. Add additional cell culture medium (e.g., 5 ml depending on expected cell number and desired cell concentration).
7. Place the cells in a CO2 incubator for one hour to overnight. Leave the cap slightly open.
Tip: Utilize this one hour of downtime to wash and block your ELISpot/FluoroSpot plate.
8. After incubation (cell resting), resuspend the cells and let any aggregated cell debris sediment (takes around 1 minute). Then carefully transfer the cell suspension, without the debris, to a fresh 15 ml tube.
Tip: Add a maximum of two cryotubes to each 15 ml tube (or around 30-40 million cells).
9. Count the cells and determine cell viability. Counting can be done with an automated cell counter or trypan blue staining using a microscope. Because only living cells will be able to secrete the analyte, make sure to exclude dead and, if possible, apoptotic cells from the cell count. If the cell concentration turns out to be lower than required, centrifuge the cells again, resuspend, and dilute to desired volume.
Video tutorial
Ok, now maybe you've already carefully read the full protocol above, but if you also want to see it in action, check out our video tutorial.